Clusterin Enhancer Alzheimer’s Therapy: A Small-Molecule Approach
Researchers have identified a clusterin enhancer Alzheimer’s therapy based on a small-molecule drug candidate that boosts levels of a naturally protective brain protein and restores memory in Alzheimer’s disease (AD) mouse models. This discovery offers a promising alternative to traditional amyloid- or tau-centric treatment strategies.
Why Clusterin Matters in Alzheimer’s Disease
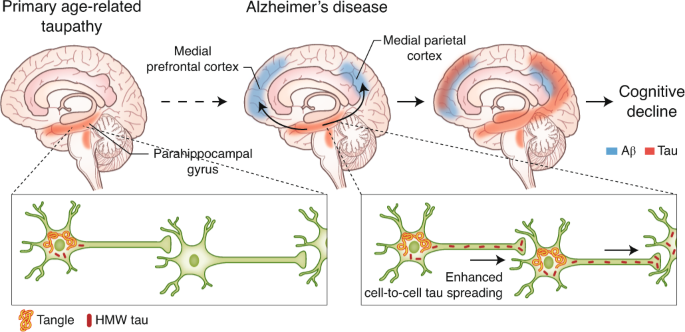
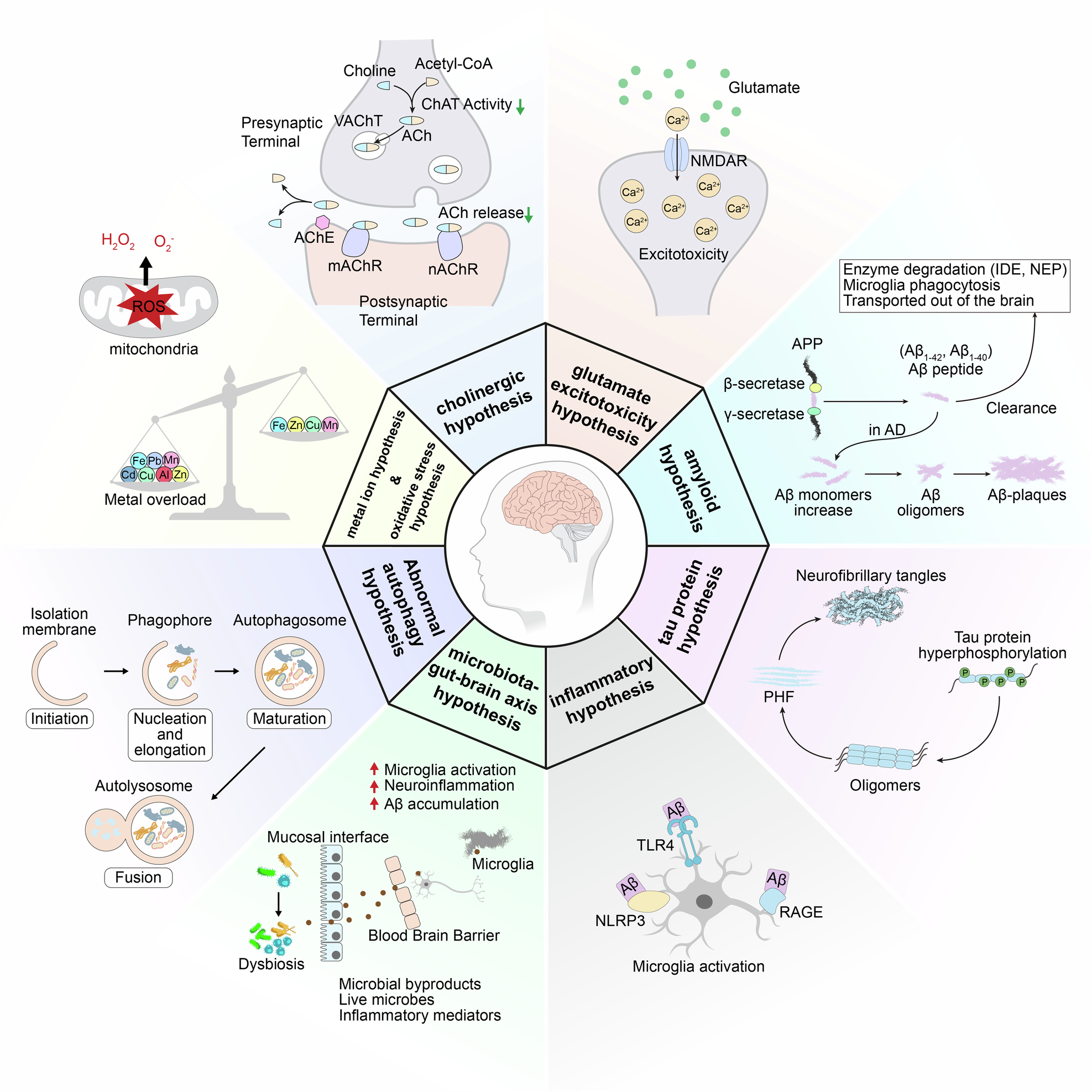
The protein clusterin—particularly its secreted isoform, secreted clusterin (sCLU)—plays a key protective role in the brain. It functions as a molecular chaperone, helping prevent the formation and accumulation of toxic protein aggregates, including amyloid-beta (Aβ) and tau, which are central pathological features of Alzheimer’s disease.
Genetic evidence strongly supports clusterin’s relevance. Variants in the CLU gene are among the most significant genetic risk factors for late-onset Alzheimer’s disease, indicating that reduced clusterin expression or dysfunction may contribute directly to disease progression.
Because of this, a clusterin enhancer Alzheimer’s strategy aims to strengthen the brain’s own protective mechanisms rather than simply targeting toxic proteins after damage has already occurred.
Discovery of a Small-Molecule Clusterin Enhancer
In their latest study, researchers at UCLA Health conducted a high-throughput screen of a large compound library using a customized assay designed to detect secreted clusterin release from cultured human glial cells.
This screening effort identified several hit compounds that increased sCLU secretion. Through medicinal chemistry optimization—focused on potency, brain permeability, and safety—the researchers developed a lead compound, DDL-357, a bromodomain and extra-terminal (BET) protein inhibitor.
The optimized molecule demonstrated favorable drug-like properties, including oral bioavailability and effective penetration of the blood–brain barrier in rodent models, supporting its potential as a small-molecule clusterin enhancer for Alzheimer’s disease.
Effects of the Clusterin Enhancer Alzheimer’s Approach in Mouse Models
In a subchronic study using the ApoE4TR-5XFAD Alzheimer’s mouse model, treatment with DDL-357 led to a measurable increase in sCLU protein levels within the brain.
More pronounced effects were observed in a chronic study using the 3xTg-AD mouse model. In this model, the clusterin enhancer Alzheimer’s therapy reduced levels of phosphorylated tau (p-tau), a neurotoxic tau species strongly associated with neurodegeneration. Treated mice also showed significant improvements in memory performance on the Barnes maze compared with untreated controls.
Proteomic analyses of brain tissue revealed additional benefits. Proteins involved in mitochondrial function, synaptic plasticity, and neuronal energy metabolism were favorably altered, consistent with improved neuronal health and synaptic connectivity.
Why a Clusterin Enhancer Could Change Alzheimer’s Treatment
Functional restoration rather than delay
Unlike therapies designed only to slow disease progression, a clusterin enhancer Alzheimer’s approach may help restore lost cognitive function.
Multi-pathway neuroprotection
By acting as a molecular chaperone, clusterin supports protein homeostasis, limits aggregation, promotes clearance mechanisms, and protects synapses—addressing several disease pathways simultaneously.
Genetics-informed therapeutic strategy
Given the strong genetic association between CLU variants and sporadic Alzheimer’s disease, enhancing clusterin may be particularly beneficial for genetically at-risk individuals.
Practical small-molecule drug design
Orally available, brain-penetrant small molecules like DDL-357 offer significant advantages over biologics, including easier administration and greater suitability for long-term treatment.
Challenges and the Road Ahead
Preclinical testing remains the major limitation. This clusterin enhancer Alzheimer’s therapy has not yet been evaluated in humans, and its safety profile, dosing parameters, and pharmacokinetics remain unknown.
Because DDL-357 functions via epigenetic modulation as a BET inhibitor, potential off-target effects must be carefully assessed. Alzheimer’s disease is multifactorial, and clusterin enhancement may ultimately be most effective in combination with other therapeutic strategies.
Conclusion: Toward a New Class of Alzheimer’s Therapies
The identification of a small-molecule clusterin enhancer Alzheimer’s therapy that increases secreted clusterin and restores memory in mouse models represents an important shift in Alzheimer’s research. By leveraging endogenous neuroprotective mechanisms rather than focusing solely on toxic protein removal, this approach moves the field toward neuroprotection and functional restoration.
If future studies confirm safety and efficacy in humans, small-molecule clusterin enhancers such as DDL-357 could become part of a new generation of accessible, multifaceted Alzheimer’s treatments with the potential to reverse aspects of cognitive decline.
Reference
Cohn W., Campagna J., Wi D., et al. Discovery of a small molecule secreted clusterin enhancer that improves memory in Alzheimer’s disease mice. npj Drug Discovery (2025). DOI: 10.1038/s44386-025-00009-2