New Biocompatible Frameworks: Small-Molecule HOFs Open the Door to Advanced Bio-Applications
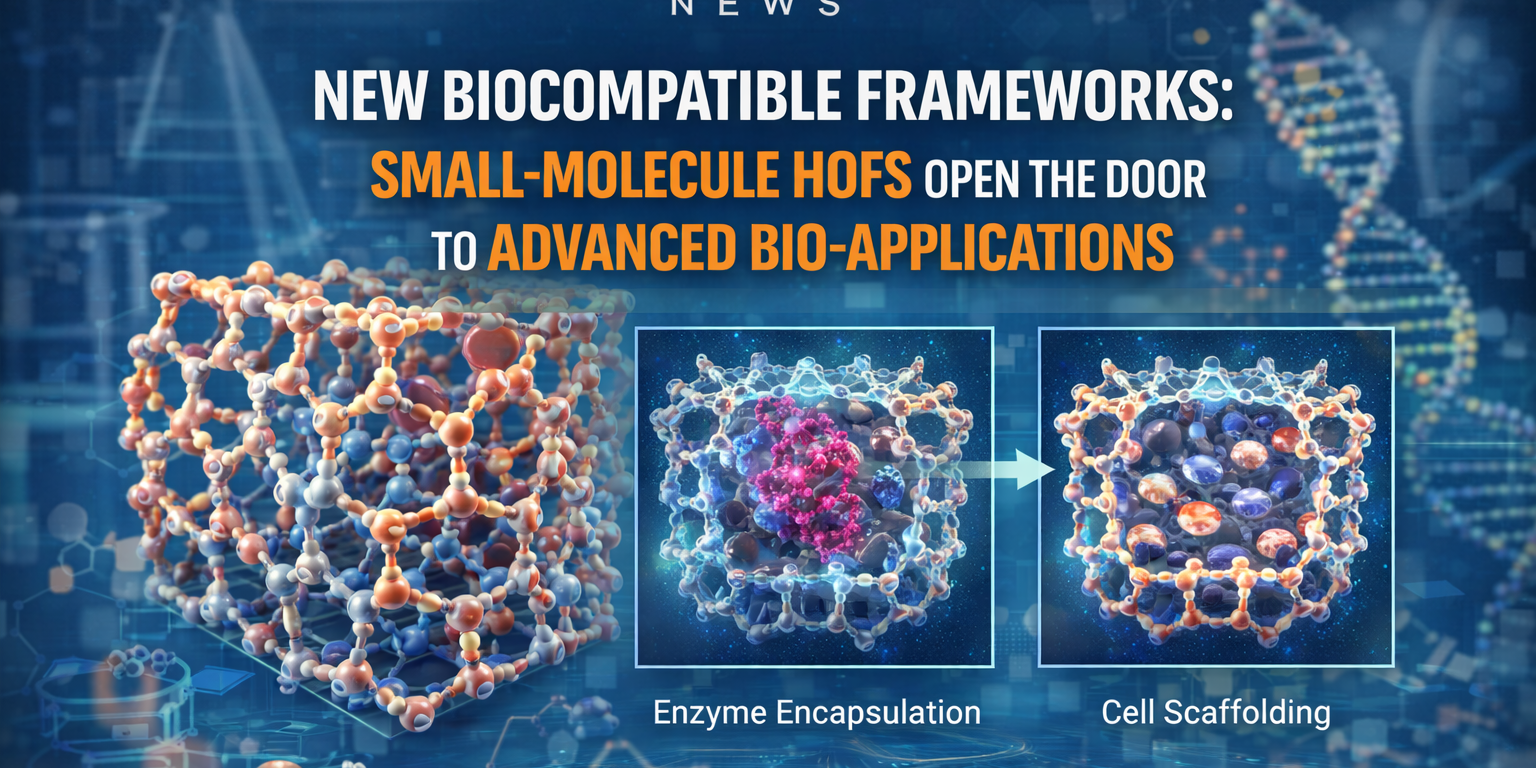
In a notable advance that bridges chemistry, materials science, and biotechnology, researchers have developed a new class of highly ordered, macroporous frameworks constructed from small, biocompatible molecules. These frameworks — a type of Hydrogen‑bonded Organic Frameworks (HOFs) — combine structural robustness, large pore sizes, and biocompatibility, making them promising substrates for enzyme encapsulation, cell scaffolding, and other bio-medical applications.
What Makes These New HOFs Special
Traditional porous materials (such as MOFs or covalent frameworks) often rely on strong covalent or coordination bonds. Weak non-covalent interactions — such as hydrogen bonding — are more challenging to harness for robust, large-pore, stable frameworks because assemblies tend to collapse once templates are removed.
In this new work, the authors overcame this limitation by choosing small, biocompatible building blocks that feature multiple hydrogen-bond donor/acceptor sites plus extended π-conjugation. This design enables the molecules to self-assemble into ordered crystalline networks in the presence of a sacrificial template, and — crucially — to retain structural integrity after template removal.
In other words: the researchers succeeded in converting dense hydrogen-bonded crystals into macroporous HOFs — a transformation that had long been difficult to realize. The resulting materials combine the advantages of small-molecule chemistry (low toxicity, affordability, tunability) with the high porosity and structural order typical of networks built via stronger bonds.
Demonstrated Utility: Enzyme Loading & Cellular Scaffolds
To demonstrate practical utility, the authors loaded the enzyme Trypsin into the new HOFs. The porous network allowed the enzyme to be encapsulated effectively without loss of function. More remarkably, when used as a scaffold with human immune-derived cells (peripheral blood mononuclear cells), the HOFs promoted differentiation into fibrocytes — evidence that the material is not only non-toxic but biologically active and supportive of cell growth and differentiation.
This proof-of-concept suggests potential applications in biocatalysis, tissue engineering, drug delivery, or cell-based therapies — areas where combining biocompatibility, porosity, and structural control is of critical importance.
Why This Matters — Broadening the HOF Landscape
Accessible and Biocompatible: Because the building blocks are small and non-toxic, these HOFs stand out as more biologically compatible than many traditional frameworks.
Scalable and Affordable: Small-molecule-based synthesis is often cheaper and easier to scale than frameworks relying on expensive ligands or metals.
Versatile Functionality: The ability to load enzymes or cells suggests broad adaptability — from bioreactors to tissue scaffolds, or even as delivery matrices.
Material Tunability: The modular, molecular nature of the building blocks means chemists may be able to design HOFs tailored for specific pore sizes, mechanical properties, or biomolecule compatibility.
Challenges and Future Directions
While this work represents a major advance, several questions remain:
Long-term Biostability & Clearance: For in vivo or therapeutic applications, the longevity of HOFs in biological environments, potential immunogenicity, and degradation/clearance must be assessed.
Functional Optimization: For enzyme- or cell-based therapies, optimizing loading capacity, diffusion rates, and retention of activity under physiological conditions will be crucial.
Scaling Production & Regulatory Hurdles: Translation from lab-scale to large-scale manufacturing — with consistent quality and purity — is often challenging, especially for medical-grade materials.
Versatility Across Cell Types and Biomolecules: Further studies are needed to test whether HOFs can support a variety of cell types (e.g., stem cells, primary differentiated cells) and different therapeutic molecules (proteins, nucleic acids, small-molecule drugs).
Conclusion — A Promising Platform for Bio-Material Innovation
By harnessing small, biocompatible molecules and clever self-assembly via hydrogen bonds, the new macroporous HOFs represent a compelling new platform for biotechnology and medicine. Their combination of porosity, structural order, and biological compatibility could make them a valuable component of next-generation biocatalysis systems, cell-scaffolding materials, or therapeutic delivery platforms.
As research progresses, these frameworks may help bridge the gap between advanced materials chemistry and practical biomedical applications — potentially enabling new therapies, regenerative medicine solutions, or biotechnological tools.
Reference
Li Q.-X., Cai W.-Z., Ye X.-L., Zeng Y., Shaheer A. R. M., Ye Z.-S., Liu T.-F. “Highly ordered macroporous hydrogen-bonded organic frameworks based on small biocompatible molecules.” Nature Communications (2025).